The
Science Notebook
Gilbert Chemistry - Part 5
The
Science Notebook
Gilbert Chemistry - Part 5
NOTE: This book was published in 1936 as a manual
to accompany several Gilbert Chemistry sets of the time.
While some of the experiments and activities here
may be safely done as written, a number of them use chemicals
and methods no longer considered safe. In
addition, much of the information contained in this book about
chemistry and other subjects is outdated and inaccurate.
Therefore, this book is probably best appreciated for
its historical value rather than as a source for current
information and good experiments. If you try
anything here, please understand that you do so at
your own risk. See our Terms of Use.
Pages 81- 100
GILBERT
CHEMISTRY 81
EXPERIMENT 134 - How to test for
soluble sulphates in water
Add two measures of strontium nitrate to a test tube one-third full
of water and shake until dissolved. If after 10 or 20 minutes the
water takes on a cloudy appearance, there are soluble sulphates
present in the water.
EXPERIMENT 135 - How to test for
lime in water
Add one or two measures of sodium carbonate to a test tube one-half
full of the water to be tested and allow to stand for 10 or 20
minutes. If the water becomes cloudy or turbid, lime is present.
EXPERIMENT 136 - How to test for
iron in water
Add one measure of sodium ferrocyanide to a test tube one-half full
of water to be tested and shake until all is dissolved. A blue
precipitate or blue color which may form at once or after standing
for several minutes indicates the presence of iron in the water.
EXPERIMENT 137 - How to test for
carbon doxide in water
Add a few drops of clear lime water to a test tube three-quarters
full of water to be tested. A white precipitate or a milky
color is a test for carbon dioxide.
Lime water is made by adding one measure of calcium oxide to a test
tube one-half full of water, shaking well and allowing any solid
material to settle. The clear liquid is lime water.
Common soda water such as is served at a soda fountain is
simply pure water which has been saturated under pressure with
carbon dioxide. When the pressure is relieved from such water the
carbon dioxide bubbles out, producing what is known as
effervescence.
SULPHUR
Sulphur is a very important element commercially, and plays a very
significant part in the physiological processes of animal
life. It is a yellowish, tasteless solid and is practically
odorless. The odor commonly ascribed to sulphur is not that of
sulphur itself, but is due to sulphur dioxide when sulphur undergoes
oxidation. The odor of burning sulphur is due to sulphur
dioxide.
In the free state sulphur occurs chiefly in volcanic regions.
Large deposits are found in Italy, Sicily, China, Ireland and India.
Important deposits are found in this country in Louisiana and
California. In Louisiana the sulphur is melted under ground by
means of superheated steam and forced out under pressure through
pipes. Sulphur also occurs in many important ores as
sulphides, for example, galena or lead sulphide; cinnabar or mercury
sulphide; zinc blende or zinc sulphide; realgar or arsenic sulphide,
and in pyrite (iron sulphide).
Sulphur is used extensively in the commercial manufacture of many
substances, such as gunpowder, fireworks, matches, dyestuffs,
medicinal products, or drugs and fertilizers. Without doubt
the most important chemical containing sulphur, which is
manufactured, is sulphuric acid. It occupies a key position in
chemical industry, and is utilized in hundreds of manufacturing
operations. Sulphuric acid is consumed in enormous quantities
in the commercial processes applied in the vulcanization of rubber,
in the bleaching industry, and in the manufacture of disinfectants
and insecticides. The great demand for insecticides by growers
of fruit and truck garden products has led to the study of many
materials to be used for plant protection. Today this
represents an enormous industry and there is a constant search for
effective chemical insecticides. Sulphuric acid is a valuable
intermediate in their manufacture.
82
GILBERT CHEMISTRY
EXPERIMENT 138 - Behavior of
sulphur at different temperatures
Heat 10 measures of sulphur in a small, dry test tube. Apply the
heat very slowly and notice the different changes. First, the
sulphur melts to a light straw colored liquid. Pour a little
of this liquid into a glass of water, and then continue the heating
of the tube, and observe, second, the change of color to brownish
black and the liquids becoming almost solid. On further heating,
third, this solid becomes liquid again. Pour this liquid sulphur
into another glass of water.
The sulphur obtained when the straw colored liquid was poured into
water is called rhombic sulphur, while that formed when the dark
black liquid was poured into water is called plastic sulphur or
elastic sulphur. This substance becomes brittle on standing for a
few days. Sulphur undergoes three distinct changes then in heating
and each change corresponds to a certain temperature.
EXPERIMENT 139 - Preparation of
lime-sulphur solution
Put into a test tube one-third full of water one measure of calcium
oxide and one measure of sulphur. Heat the test tube over a
flame and boil for several minutes. Notice the yellow colored
solution that is formed. This solution is known as lime
sulphur solution and is used on a large scale for spraying fruit
trees and destroying fungi.
The calcium oxide reacted with the sulphur to form calcium sulphide,
which is soluble in the water. Filter a part of the calcium
sulphide solution, and to the clear fluid add acetic acid until the
solution is acid to litmus paper. There will be an immediate
evolution of hydrogen sulphide, which is evidenced by the odor.
Allow 1 or 2 drops of the calcium sulphide solution to fall on
a polished silver coin, and let stand for a few minutes. Then
wash the coin with water and notice that a black spot of silver
sulphide is formed. Exposure of silverware to eggs will
produce a similar discoloration due to the presence of sulphur in
eggs.
EXPERIMENT 140-Sulphur dioxide from
burning sulphur
Put 2 measures of sulphur in the spoon and heat over the flame.
The sulphur will suddenly take fire and burn with a blue
flame. The gas produced, having a suffocating odor, is sulphur
dioxide, and is formed by the oxidation of sulphur when it burns in
the air.
EXPERIMENT 141 - Sulphur dioxide
from sodium bisulphite
Put two measures of sodium bisulphite in a test tube one-third full
of water and add a few drops of acetic acid. Smell cautiously
at the mouth of the tube and notice the smell of burning sulphur.
Sodium bisulphite when treated with an acid reacts to form sulphur
dioxide, water and a salt.
EXPERIMENT 142-Bleaching with
sulphur dioxide
Put five measures of sodium bisulphite in a glass tumbler.
GILBERT
CHEMISTRY 83
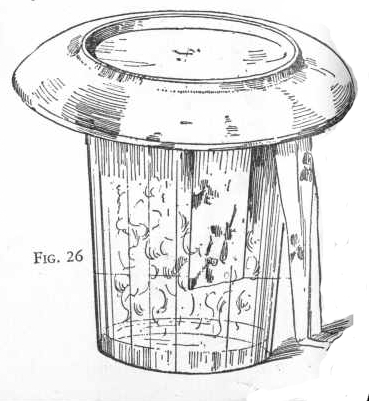
Now obtain some colored flowers or pieces of dyed cloth and moisten
them with water. Put a teaspoonful of vinegar in the tumbler
and quickly introduce the flowers or pieces of colored cloth. Place
a saucer over the mouth of the tumbler and set aside for one-half
hour. Figure 26.
Notice the vigorous reaction which takes place when the vinegar is
added. This is due to the liberation of sulphur dioxide gas. At the
end of a half hour examine the flowers or cloth and notice that some
of the colors have been bleached out. Sulphur dioxide will bleach
certain colors but not all colors.
Sulphur dioxide is used commercially in bleaching straw, silk and
woolen goods, or any material that would be injured
by chlorine.
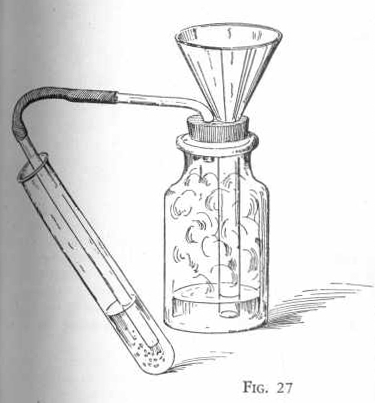
EXPERIMENT 143-Preparation of
sulphurous acid
Put six measures of sodium bisulphite in the gas generator flask set
up as shown in Figure 27 and add about one-half inch of water. Place
over the end of the delivery tube a test tube one-half full of water
so that the end of the delivery tube just extends below the surface
of the water in the test tube.
Now add a little acetic acid, two or three drops at a time, through
the funnel which must extend below the surface of the liquid in the
generator flask. Notice the action which takes place. After passing
the gas, which is formed in the reaction, into the test tube for
several minutes, remove the test tube. Test the liquid in the test
tube with blue litmus paper and notice that the litmus paper turns
red, proving that an acid has been formed. This acid is
sulphurous acid.
The acetic ac1d reacted with the sodium bisulphite to form sulphur
dioxide gas. This gas when passed into the test tube of water
reacted with the water to form suphurous acid.
We have shown that when sulphur is burned in the air or when sodium
bisulphite is treated with an acid sulphur dioxide is formed.
Now in the presence of an oxidizing agent sulphur dioxide can
be made to combine with one more atom of oxygen to form sulphur
trioxide.
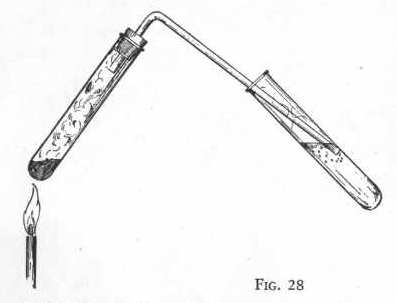
EXPERIMENT 144 - Preparation of
sulphur trioxide.
Put three measures of sodium bisulphite and two measures of
potassium permanganate in a clean, dry test tube and shake the tube
until the two substances are thoroughly mixed. Now insert the
gas delivery tube and put the end of the delivery tube just below
the surface of the water in another test tube one-third full of
water. Figure 28.
84
GILBERT CHEMISTRY
Now heat slowly the test tube containing the mixture of the two
compounds, being careful that the water in the second test tube does
not suck back into the first test tube. To prevent this remove
the delivery tube from the tube containing the water when you stop
heating. After passing the gas into the water for a few moments
remove the test tube containing the water and and test the water
with a piece of blue litmus paper. Notice that it turns red, proving
that an acid is formed. This acid is sulphuric acid. Add a few drops
of a solution of barium salt and note the result. Explain.
Sodium bisulphite when heated in the presence of an oxidizing agent
like potassium permanganate forms sulphur dioxide, which is readily
oxidized to sulphur trioxide. This oxide when passed into water
forms sulphuric acid.
Sulphuric acid is really a very important acid. The reactions
involved in the manufacture of this acid are more complicated than
those of the manufacture of the other acids because it must be built
up from its elements. Sulphuric acid is made commercially by either
one of two methods.
First, the "Lead Chamber Process" or the older method. In this
process sulphur dioxide is prepared by burning sulphur or an ore of
sulphur such as iron sulphide or pyrite. The sulphur dioxide is then
conducted into large lead chambers where it comes in contact with
oxygen, oxides of nitrogen and steam. These react to form
sulphuric acid which is concentrated by heat treatment.
The second method, known as the "Contact Process" is the more recent
method and gives an acid of much higher purity. This is a more
expensive method, but this is compensated for by the degree of
purity in the acid produced. In this process sulphur dioxide
is formed the same as in the lead chamber process. It is then
passed through a tube heated to 400 degrees Centigrade and
containing a substance known as a catalytic agent. The
catalytic agent has the property of making the sulphur dioxide
combine with more oxygen to form sulphur trioxide and this is passed
into water forming concentrated sulphuric acid.
EXPERIMENT 145-Preparation of
sulphuric acid
Mix together on a piece of paper 1/2 measure of sulphur and 1/2
measure of potassium nitrate. Put 1/4 of this' mixture - no
more - in a clean, dry test tube and heat slowly over a flame.
Notice the white fumes which are given off. These fumes are sulphur
trioxide. After the fumes stop coming off, stop the heating and
place the thumb over the mouth of the tube.
After the tube has become cold, fill the test tube 1/2 full of water
and shake the test tube, holding the thumb over the mouth. Test the
liquid with blue litmus paper and notice that it turns red. Sulphur
trioxide combined with the water to form sulphuric acid.
GILBERT
CHEMISTRY 85
EXPERIMENT 146-Sulphuric acid from
sulphur dioxide and hydrogen peroxide
Fit up your gas generator bottle as previously directed, but fill
the test tube half full of hydrogen peroxide solution (dioxygen).
Now generate sulphur dioxide by adding a little acetic acid, two or
three drops at a time, through the funnel in the generator flask and
pass the gas over into the hydrogen peroxide solution for three or
four minutes.
Now remove the test tube from the delivery tube and test the
solution with a piece of blue litmus paper. Notice that it turns
red, proving that an acid was formed. This acid is sulphuric acid.
Sulphur dioxide was formed by the action of acetic acid upon sodium
bisulphite, and sulphur dioxide when passed into hydrogen peroxide
solution is oxidized to sulphur trioxide. Sulphur trioxide
reacted with water in the hydrogen peroxide solution to form
sulphuric acid.
Sulphates are salts of sulphuric acid, and many of them find use in
commerce. These salts are all soluble in water except those of
barium, strontium and lead. The chemist makes use of this
insolubility of their sulphates in precipitating these metals from
their solutions.
EXPERIMENT 147 - How to make
strontium sulphate
Dissolve one measure of aluminum sulphate in a test tube one-quarter
full of water. In another test tube one-quarter full of water
dissolve two measures of strontium nitrate. Now mix the two
solutions and notice the white precipitate of strontium
sulphate which is formed.
Aluminum sulphate reacts with strontium nitrate to form a soluble
compound of aluminum nitrate and an insoluble compound of strontium
sulphate.
HYDROGEN
SULPHIDE
Hydrogen sulphide (H2S) and water (H2O) are
members of the same chemical family. Water is a neutral
substance, while hydrogen sulphide is a weak acid. The salts of
hydrogen sulphide are called sulphides. Some sulphides are very
insoluble in water and for that reason find application by chemists
in analytical work. Certain metals can be separated from each other
by means of their sulphides.
EXPERIMENT 148-How to make hydrogen
sulphide
Cut a piece of paraffin from a candle or a piece of paraffin wax
about the size of a pea and put it in a test tube. Add 2 measures of
sulphur, place a piece of moistened sulphide test paper over the
mouth of the tube and heat the tube slowly. Notice that the test
paper turns black This is a test for hydrogen sulphide gas.
The test paper contains lead acetate and when the hydrogen sulphide
comes in contact with it, it forms a black precipitate of lead
sulphide.
Remove the tube from the flame and smell cautiously at the mouth of
the tube. Note the resemblance of the odor to that of rotten eggs.
As a matter of fact this is the gas produced when eggs go bad.
Hydrogen sulphide is given off from several organic compounds, for
example, when cabbage is cooked.
Hydrogen sulphide is inflammable and when burned the hydrogen
combines with oxygen to form water while the sulphur combines with
oxygen to form sulphur dioxide.
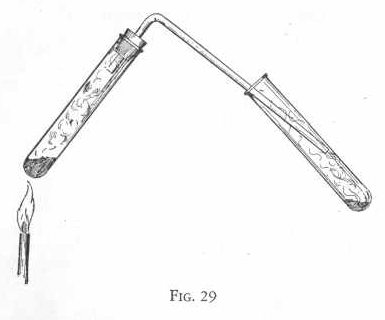
EXPERIMENT 149 - Action of
hydrogen sulphide on sulphur dioxide
Prepare hydrogen sulphide gas as in preceding experiment, using five
measures of sulphur instead of two measures.
86
GILBERT CHEMISTRY
Now pass this gas by means of a delivery tube into a test tube
containing two measures of sodium bisulphite and six or eight drops
of vinegar or acetic acid. (Figure 29.) Notice that a deposit
of sulphur is formed around the inside of the test tube containing
the sodium bisulphite and vinegar.
Sulphur dioxide was formed in the second test tube by the action of
the vinegar or sodium bisulphite and vinegar. This reacted with the
hydrogen sulphide gas to form su1phur and water. This is the
way sulphur is formed around many volcanic regions.
EXPERIMENT 150 - Action of chlorine
gas on hydrogen sulphide
Prepare a solution of hydrogen sulphide water by bubbling hydrogen
sulphide gas into water.
Now prepare some chlorine gas by putting five measures of tartaric
acid and three measures of calcium hypochlorite in a test tube
one-quarter full of water. Attach the gas delivery tube to
this test tube and allow the gas which comes off to bubble through
the hydrogen sulphide water prepared above. Notice that a white
precipitate is formed. This is sulphur.
Chlorine reacts with hydrogen sulphide to form hydrochloric acid and
sulphur.
EXPERIMENT 151 - Reducing action of
hydrogen sulphide
Dissolve a crystal of potassium permanganate in a test tube half
full of water.
Now bubble hydrogen sulphide gas, prepared as described in a
previous experiment, using a gas delivery tube, into the solution of
potassium permanganate and notice that the purple color fades
and a white precipitate of sulphur is formed.
Hydrogen sulphide gas reduced the potassium permanganate, therefore
removing its color. In reducing the permanganate it is
oxidized by oxygen in the permanganate to water and sulphur.
Hydrogen sulphide gas is often used as a reducing agent, and
the two preceding experiments with chlorine and hydrogen peroxide
illustrate this behavior
You probably have noticed that some white paints turn black after
exposure for a long time. This is because such paints contain
lead, chiefly in the form of the pigment lead carbonate. The lead
reacts with traces of hydrogen sulphide gas in the air to form black
lead sulphide. To prevent this discoloration, zinc oxide is
used as a base pigment in place of lead carbonate. When zinc oxide
reacts with hydrogen sulphide a white precipitate of zinc sulphide
is formed.
EXPERIMENT 152-How to restore the
color of white paint
Obtain some white oil paintings which have become dark by the action
of hydrogen sulphide. Wash these paintings with a little hydrogen
peroxide solution and notice that they become white
again.
GILBERT
CHEMISTRY 87
EXPERIMENT 153 - Silver sulphide
Place 1/2 measure of sulphur on a bright silver coin and wrap in
several thicknesses of paper. After a few days you will find a
black spot of silver sulphide on the coin where the sulphur was in
contact with it.
EXPERIMENT 154 - Sulphur in rubber
Rubber contains sulphur used in its vulcanization. Wrap a
rubber band around a silver coin and you will find that it will turn
black after a few days, due to the formation of silver sulphide.
EXPERIMENT 155 - Sulphur and silver
coin
a silver coin is turned black in a few hours by a paste of mustard
and water, as mustard contains sulphur. Eggs also contain
sulphur, and this is the reason why silver spoons turn black when
used for eating eggs.
EXPERIMENT 156 - Nickel sulphide
Dissolve one measure of nickel ammonium sulphate in a test tube
one-quarter full of water. Fill another test tube one-quarter
full of water and add four measures of sodium bisulphate. Shake to
dissolve the sodium bisulphate and add two measures of iron
sulphide. You will find this in any drug store. Fit the
test tube with the gas delivery tube and stopper and when hydrogen
sulphide gas is coming off freely, allow it to bubble through the
nickel ammonium solution. ln a few minutes you will notice a
black precipitate of nickel sulphide forming in the nickel ammonium
sulphur solution.
EXPERIMENT 157 - Copper sulphide
Place one measure of copper sulphate in a test tube and fill the
tube half full of water, warm the mixture gently until the solid is
dissolved. Now cool the solution and pass in hydrogen sulphide
gas through the gas delivery tube as in the preceding
experiment. A black precipitate of copper sulphide will be
formed.
EXPERIMENT158 - Zinc
sulphide
Place 2 measures of sodium bisulphate and a small piece of zinc
metal in a test tube and fill the tube 1/4 full of water. Heat the
tube gently and wait until some of the zinc has dissolved. Now pour
some of the clear solution into another test tube, add water to fill
the tube one-half full and pass in hydrogen sulphide gas.
EXPERIMENT 159 - Ferrous sulphide
from sodium thiosulphate
Dissolve one measure of ferrous ammonium sulphate in a test tube
one-quarter full of water. Add one measure of sodium
thiosulphate and heat the solution for a minute or two. A
greenish brown precipitate of ferrous sulphide will form.
EXPERIMENT 160 - Ferric sulphide
from sodium thiosulphate
Add one measure of ferric ammonium sulphate in a test tube
one-quarter full of water. Add one measure of sodium
thiosulphate and heat the solution. This time a brown precipitate
will form.
EXPERIMENT 161 - Nickel sulphide
from sodium thiosulphate
Dissolve one measure of nickel ammonium sulphate in a test tube
one-quarter full pf water. It may be necessary to warm the
tube slightly to completely dissolve the nickel ammonium sulphate.
Now add one measure of sodium thiosulphate and the black
precipitate, consisting of nickel sulphide, will be formed as soon
as the solution is heated.
88
GILBERT CHEMISTRY
EXPERIMENT 162-Manganese sulphide
from sodium thiosulphate
To form a white precipitate of manganese sulphide, dissolve one
measure of manganese sulphate in a test tube one-quarter full of
water. Add one measure of sodium thiosulphate; warm the solution
gently and the white precipitate will form.
THE
HALOGENS
The elements that go to make up the halogen family are fluorine,
chlorine, bromine and iodine. These elements are called halogens,
meaning salt producers. They all resemble each other very much in
chemical properties but differ widely in physical properties.
Fluorine is a colorless gas, chlorine a greenish yellow gas, bromine
a brownish-red liquid and iodine a purple black solid.
The halogens are very active substances, so that they never occur in
the free state in nature. Their compounds are very abundant - those
of chlorine, bromine and iodine occurring in sea water. The
most common of these is sodium chloride or common salt.
As already stated, the halogens are very active substances.
They combine with metals like copper, sodium, potassium, gold,
silver, platinum, etc., to form salts of these metals.
They also react with non-metals like sulphur, antimony and
arsenic to form compounds with these substances. They also
react with hydrogen to form the corresponding acids, namely,
hydrofluoric, hydrochloric, hydrobromic and hydriodic acids.
Of the halogens, fluorine is the most active and iodine the least
active. All four of the halogens find wide commercial
applications.
Chlorine is used extensively as a bleaching agent and germicide.
Chlorine gas is shipped in bulk compressed in iron cylinders. The
gas is widely used for water purification. It also comes on
the market known as bleaching powder or chloride of lime. The
corresponding acid, hydrochloric acid, is an important technical
acid and is used for a number of purposes.
Bromine is used principally in the preparation of bromides, which
are used to a considerable extent in photography and in medicine.
lt is also used in the preparation of a number of organic
drugs and dyestuffs. Bromine is extracted today in large
quantities from sea water and apparently this source is
inexhaustible.
The chief sources of iodine are brine wells and the ashes of certain
sea weeds. Iodine is used extensively in medicine, especially
in the form of tincture of iodine. It also finds an important
use in the preparation of iodides and of certain dyes and
drugs. The antiseptic iodoform is a compound of iodine with
carbon and hydrogen. This iodine compound is analogous to the
widely used anesthetic - chloroform - which is a compound of
chlorine combined with hydrogen and carbon. While iodoform and
chloroform are valuable drugs, the corresponding compounds of
bromine and fluorine are unimportant compounds in medicine.
EXPERIMENT 163 - How to make
chlorine gas
Put two measures of potassium nitrate, two measures of sodium
bisulphate and two measures of sodium chloride (common table salt)
in a test tube and heat the test tube gently over a flame for a
few moments. Remove the test tube from the flame and smell
cautiously the gas which is given off. This is chlorine
gas.
Sodium bisulphate reacted with sodium chloride to form hydrogen
chloride gas which was oxidized by oxygen from the potassium nitrate
to water and chlorine gas.
EXPERIMENT 164 - To show the
bleaching properties of chlorine
Prepare chlorine gas as in the preceding experiment, placing a small
piece of moistened blue litmnus paper over the mouth of the tube
before heating. Notice on
GILBERT
CHEMISTRY 89
heating that the blue litmus paper turns white, showing that
chlorine gas has the property of bleaching certain colors.
What happened was that the chlorine gas reacted with the water on
the blue litmus paper forming hydrochloric acid and oxygen. It is
really this free oxygen that does the bleaching.
EXPERIMENT 165 - How to make
hydrochloric acid
Put two measures of ammonium chloride and two measures of sodium
bisulphate in a test tube. Moisten a piece of blue
litmus paper and place it over the mouth of the test tube. Now
heat the tube slowly over a flame for a few minutes. Notice
that the litmus paper turns red, proving that an acid has been
formed. Remove the test tube from the flame and smell cautiously the
gas that is given off. This is hydrogen chloride gas.
Dip the glass stirring rod in a little household ammonia and hold
the rod over the mouth of the test tube. Notice the white
fumes that are formed. These fumes are ammonium chloride
fumes.
Hydrogen chloride gas, as prepared in this experiment, when
dissolved in water forms hydrochloric or muriatic acid.
Commercially, hydrochloric acid is manufactured by heating
sodium chloride with sulphur acid.
BLEACHING
POWDER
Bleaching powder or "Chloride of Lime," as it commonly comes on the
market, is a compound composed of calcium, oxygen and chlorine. It
is prepared by passing chlorine gas over slaked lime, bleaching
powder or calcium hypochlorite being formed.
Bleaching powder is a very important compound and has many uses in
everyday life. It is a very good bleaching agent and readily
gives up its chlorine when treated with an acid. In bleaching
paper rags, the rags are first boiled in an alkali to remove the
grease, then placed in a large vat with bleaching powder and
sulphuric acid; on removing, the rags are pure white. Cotton
cloth is bleached by passing it through alternate vats of bleaching
powder and sulphuric acid.
Besides being used as a bleaching agent chloride of lime is employed
as a disinfectant, since it destroys germs. Water is sometimes
purified with this compound.
EXPERIMENT 166 - How to make
chlorine from bleaching powder
Make a solution of tartaric acid in water by adding 3 measures of
tartaric acid to a test tube containing about 1/2 inch of water.
Then add 3 measures of calcium hypochlorite or bleaching powder and
smell cautiously at the mouth of the tube. Note the odor of
chlorine gas. The other compound formed in the reaction is calcium
tartrate.
EXPERIMENT 167 - Bleaching with
bleaching powder
For this experiment obtain two or three pieces of colored cloth and
some colored flowers; a red carnation works well.
Put 5 measures of calcium hypochloride (bleaching powder) and 5
measures of tartaric acid in a clean glass and add a few drops of
water to moisten the compounds. Then moisten the pieces of
colored cloth and flowers with water, place them in the glass and
cover the glass with a saucer. Allow the glass to stand
for an hour. Notice that the cloth and the flowers lose their
color, and become white, due to the bleaching effect of the chlorine
gas liberated in the reaction.
EXPERIMENT - 168 How to make iodine
Put 4 drops of sodium iodide solution into a test tube 1/4 full of
water and mix the contents of the tube by shaking.
90
GILBERT CHEMISTRY
Now prepare chlorine gas as already shown in a previous experiment
and fit the test tube with a gas delivery tube. Allow the chlorine
gas to bubble through the sodium iodide solution prepared above
and notice after a few moments that the solution turns brown.
Chlorine gas reacts with sodium iodide to form sodium chloride and
free iodine. It is this free iodine that gives the solution
its brown color.
Now add 6 or 8 drops of carbon tetrachloride to this solution
containing the iodine, close the mouth of the tube with your thumb
and shake several times. Allow the test tube to stand for a
few seconds and notice that the carbon tetrachloride has become red
and the brown color has disappeared from the water.
EXPERIMENT 169 - How to test for
iodine
Prepare some starch solution by putting 1 measure of powdered
household starch in a test tube and moisten with a few drops of
water. Now heat a test tube half full of water to boiling and
pour the hot water into the test tube containing the starch paste.
To this starch solution add 5 drops of sodium iodide solution.
To a dry test tube add 2 measures of common table salt or sodium
chloride, 2 measures of potassium nitrate and 2 measures of sodium
bisulphate. Insert the perforated cork with gas delivery tube and
heat the test tube slowly over a flame. After the gas starts
to come off freely insert the end of the delivery tube into the test
tube containing the starch solution and sodium iodide and allow the
chlorine gas to bubble through the starch solution for several
moments. Notice the blue color which is formed. This is
the test for free iodine.
The chlorine gas displaced the iodine in the sodium iodide solution,
liberating iodine. Free iodine in the presence of starch produces a
blue color.
EXPERIMENT 170 - Formation of lead
iodide
Fill a test tube one-third full of water and add six drops of sodium
iodide solution. In another test tube place about one measure
of lead nitrate or acetate obtainable at any drug store and dissolve
by adding one-third test tube of water. Now pour the nitrate
solution into the sodium iodide solution. A heavy bright yellow
precipitate of lead iodide will form. Now heat until it begins
to boil, then set aside to cool. On cooling watch closely what
happens. Most of the lead iodide will have settled to the
bottom, but there also appears above it, numerous little scales or
specks of all colors - some golden, some silver blue, green, red,
pink, orange, and violet which sparkle beautifully. The lead iodide,
which by this time has all settled to the bottom of the test tube,
leaves the most beautiful colored specks in the almost clear liquid
above it. Its beauty cannot be very easily described, but soon
the beautiful specks settle to the bottom of the test tube leaving
only a few lingering above them. Even at the bottom they produce a
striking effect. It is very interesting to watch the colored
particles.
EXPERIMENT 171 - Partition
solubility of iodine
To a half test tube of water add about ten drops of tincture of
iodine (this you will find in the medicine closet). The solution in
the test tube will be a reddish-brown color. Add to this an eighth
test tube of carbon tetrachloride. The carbon will sink to the
bottom. Shake the test tube and then let the carbon tetrachloride
layer settle to the bottom of the tube. The bottom layer will
be a rich violet color.
Reaction: Tincture of iodine is a solution of iodine and potassium
iodide in water and alcohol. When carbon tetrachloride is added, it
dissolves the free iodine. The carbon tetrachloride settles to the
bottom of the test tube because it is heavier than water and it is
not miscible with water.
GILBERT
CHEMISTRY 91
EXPERIMENT 172 - Testing vegetables
for starch
Make a solution of iodine by dissolving 2 crystals of iodine in a
test tube 1/4 full of water containing 5 drops of sodium iodide
solution. You will notice that the solution turns brown, showing
that iodine is soluble in sodium iodide solution.
Now cut in half a potato, beet, carrot or any other vegetable that
you may obtain and add 2 or 3 drops of the iodine solution to the
freshly cut surface. Notice which of the vegetables produce a blue
color. The potato contains a lot of starch. A more pronounced blue
color may be obtained by boiling a small piece of the vegetable and
adding 1 or 2 drops of the iodine solution to the cool solution
containing the vegetable.
EXPERIMENT 173-Testing other
substances for starch
Test some grains of corn, barley and wheat for starch the same way
you did in the preceding experiment. Notice that these substances
contain starch.
GAS
WARFARE

It was not until the last World War that poisonous gases were
demonstrated to be an important factor in modern military science.
They were introduced so unexpectedly during the critical period of
the world’s conflict that the unprepared combatants were handicapped
in military efficiency, and months of intensive training in the new
technique of chemical warfare became necessary before the different
countries were able to provide a defense against this new method of
attack. In the early development of this new method of warfare,
chlorine gas or compounds con-
92
GILBERT CHEMISTRY
taining this halogen were used extensively, and they made up the
bulk of the so-called poisonous gases. Chlorine itself is a heavy
suffocating gas which can be produced cheaply, and is easily
transported in steel cylinders. Other chlorine compounds of high
toxicity which were used in quantity as poisonous gases were
chloropicrin and phosgene. Chlorine gas was later abandoned and was
replaced by special compounds containing this element in combination
with sulphur, carbon and hydrogen, which pr proved to be more
destructive and efficient for offensive military movements. One of
the most valuable products developed for the chemical warfare
service was a chlorine compound known as "mustard gas." Large
chemical plants were operated in the United States during the World
War for the manufacture of chlorine gas, phosgene, chloropicrin, and
mustard gas, to be shipped abroad to the military forces engaged in
this destructive war.
Today poisonous gases fulfill an important commercial service in
their uses as protective measures against bank robberies, and for
police equipment in controlling public riots and serious labor
strikes. Such cases provide a more effective and humane weapon of defense than the
rifle or police gun.
THE
GAS MASK
As a preventative from gas poisoning, the well-known gas mask was
invented. This consisted of a face piece to shut out the gases
from the nose, mouth and eyes and from which ran a flexible rubber
tube to a cannister or container which held the chemical for
neutralizing the poisonous gases. On breathing in, the poisonous air
passes into the cannister where the deadly gases are removed,
allowing the good air to pass up through the flexible tube, the end
of which is held in the mouth, and then into the lungs. The
exhaled air is passed out through a rubber slit in the lower part of
the face piece. The chemicals used to remove the gases were
principally a mixture charcoal and soda lime.
BORON
AND THE BORATES
Boron does not occur as the free element. It has been found in
nature in the form of boric acid in many hot springs, particularly
in Italy and California. Boron occurs in large quantities in the
desert regions of California and Nevada in the form of its sodium
salt, known as borax, ordinary "Twenty Mule Team Borax." It also
occurs as the calcium salt.
Boric acid is used as a mild antiseptic, as a constituent of talcum
powder, and as a preservative.
EXPERIMENT 174 - Boric acid from
borax
In a test tube 1/4 full of water, dissolve 8 measures of borax
heating the tube a little to completely dissolve the solid. In
another test tube 1/4 full of water dissolve, by heating if
necessary, S measures of sodium bisulphate. Now pour the sodium
bisulphate into the borax solution and cool the resulting solution
by holding the test tube in water. After some time a white solid
crystallizes out. This solid is boric acid.
EXPERIMENT 175-Borax from boric
acid
Put 2 measures of boric acid in a test tube 1/4 full of water and
heat to boiling over a flame. Now add 3 measures of sodium carbonate
and boil the solution for two or three minutes. Set the tube aside
to cool and observe the behavior while crystals separate out. These
are crystals of sodium tetraborate or borax.
GILBERT
CHEMISTRY 93
EXPERIMENT 176 - Test for boric
acid
Dissolve 2 measures of boric acid in a test tube 1/3 full of
alcohol. Now pour this solution into a saucer, darken the room and
light the alcohol with a flame. Notice that the alcohol burns with a
green flame. This is a test for boric acid.
EXPERIMENT 177 - Examination of
talcum powder
Repeat experiment 176 using instead of the boric acid 3 or 4
measures of a talcum powder. Some talcum powders contain boric
acid.
BORAX
GLASS
When heated, borax swells up to a bulky mass, loses its water of
crystallization and then melts to a clear glass. This glass readily
dissolves various metallic oxides which impart characteristic colors
to the glass. This property is used in testing for certain
metals. For this same reason borax is used as a flux in
brazing or hard soldering to remove the oxides from the surface of
the metals to be joined.
EXPERIMENT 178 - Cobalt borax glass
Put 1 measure of borax on a clean sheet of paper and mix with it a
very small quantity (a speck about the size of a pin point) of
cobalt chloride. Now pick up some of this mixture on the loop of
your nickel steel wire and heat it over the alcohol lamp
flame. The borax will melt as before, but you will find that
the glass which is formed will be blue in color because of the
cobalt which dissolved in it. If very much cobalt is used the film
will appear to be black, so intense is the coloring power, but if
only a very slight trace of the cobalt is used the film will be a
beautiful azure blue color.
EXPERIMENT 179 - Iron borax glass
Take a fresh quantity of borax, about 1 measure, and put it on a
clean sheet of paper. Mix with the borax a very small trace of
ferric ammonium sulphate.
Now put some of this mixture on the loop of your nickel steel wire
and heat it. This time you will obtain yellow borax glass due
to the presence of iron.
EXPERIMENT 180 - Manganese borax
glass
Manganese colors borax violet or lilac. Mix a very small
amount of manganese sulphate with 1 measure of borax and make a ball
of borax glass on your nickel steel wire using this mixture.
EXPERIMENT 181 - Nickel borax glass
Nickel colors borax glass brown. Mix a very small quantity of
nickel ammonium sulphate with 1 measure of borax and make a ball of
borax glass from this mixture.
EXPERIMENT 182 - Chromium borax
glass
Chromium colors borax glass green. Mix a very small quantity of
chrome alum with 1 measure of borax. Make a ball of borax
glass from this mixture.
BORON
COMPOUNDS
EXPERIMENT 183 - Strontium borate
Dissolve 2 measures of strontium chloride in a test tube 1/2 full of
water. In another test tube 1/4 full of water dissolve 2 measures of
borax. Now pour one of these solutions into the other and
notice the heavy white precipitate of strontium borate which forms.
94
GILBERT CHEMISTRY
EXPERIMENT 184 - Magnesium borate
Dissolve 2 measures of magnesium sulphate in a test tube 1/4 full of
water. Upon pouring this into a solution of borax a white
precipitate of magnesium borate is formed.
EXPERIMENT 185 - Aluminum borate
Prepare a solution of 2 measures of aluminum sulphate in a test tube
1/4 full of water, and add to this a solution of 2 measures of
borax, dissolved in a test tube 1/4 full of water.
EXPERIMENT 186 - Ferric borate
Prepare a solution of 2 measures of ferric ammonium sulphate in a
test tube 1/4 full of water, and also a solution of 2 measures of
borax in a test tube 1/4 full of water. Upon pouring one of
these solutions into the other a heavy precipitate of ferric borate
is formed.
EXPERIMENT 187 - Cobalt borate
Dissolve 1 measure of cobalt chloride in a test tube 1/4 full of
water. In another test tube 1/4 full of water dissolve 2 measures of
borax. Pour the cobalt solution into the borate solution and a very
pretty precipitate of cobalt borate will result.
EXPERIMENT 188 - Nickel borate
To precipitate nickel borate prepare a solution of two measures of
nickel ammonium sulphate in a test tube 1/4 full of water. lt is
necessary to heat the solution a little in order to dissolve all of
the nickel ammonium sulphate. In another test tube prepare a
solution of two measures of borax and pour the nickel ammonium
sulphate solution into the borate solution. A thick greenish
precipitate of nickel borate will result.
EXPERIMENT 189 - Manganese borate
Dissolve 2 measures of manganese sulphate in a test tube 1/4 full of
water, and dissolve 2 measures of borax in another test tube 1/4
full of water. Pour the manganese solution into the borate solution
and a thick white precipitate of manganese borate will be formed.
EXPERIMENT 190 - Calcium borate
Prepare a solution of 2 measures of calcium chloride in a test tube
1/4 full of water. In another test tube dissolve 2 measures of
borax and pour the calcium chloride solution into the borax
solution.
EXPERIMENT 191 - Chromium borate
Prepare a solution of 2 measures of chrome alum in a test tube 1/4
full of water. Dissolve 2 measures of borax in another test
tube 1/4 full of water. Upon pouring these solutions together a
green precipitate of chromium borate is formed.
PHOSPHOROUS
AND THE PHOSPHATES
Phosphorus never occurs free in nature but is found is combination
with oxygen and metals as derivatives of phosphoric acid. It occurs
most extensively as calcium phosphate. All fertile soils contain
calcium phosphate. Since it is essential to plant growth, it is an
important constituent of fertilizers, the soluble calcium
monophosphate being used. The bone of animals is largely calcium
phosphate.
Phosphorus exists in two forms. Yellow phosphorus is a transparent,
wax-like solid which often takes fire in air at ordinary
temperature. It is always kept under water.
GILBERT
CHEMISTRY 95
Yellow phosphorus is very poisonous. Red phosphorus is a
chocolate red amorphous powder and is quite stable in air at
ordinary temperature.
The phosphates of most metals can be precipitated from solution of
the salts since most phosphates are insoluble in water.
EXPERIMENT 192 - Nickel phosphate
Place in a test tube 1/2 measure of sodium carbonate and 1/2 measure
of calcium monophosphate. Fill the test tube half full of
water and shake for a moment. The heavy white precipitate which is
formed will gradually settle to the bottom of the tube leaving a
clear solution on top. This solution contains sodium phosphate
which is soluble in water and the precipitate in the bottom of the
tube consists of calcium carbonate.
Now place in another test tube two measures of nickel ammonium
sulphate. Fill the test tube half full of water and heat for a few
moments to completely dissolve the solid. Cool the solution of
nickel ammonium sulphate by holding the bottom of the tube in cold
water for a moment, and then add a few drops of the clear solution
of sodium phosphate to the nickel ammonium sulphate solution. A
thick green precipitate of nickel phosphate will be formed.
The precipitate of nickel phosphate is soluble in solutions
containing ammonium salts. Place 3 measures of ammonium
chloride in a test tube. Fill the tube 1/4 full of water and shake
to dissolve the ammonium chloride. Add to this solution about 1/4 of
a test tube of the nickel phosphate precipitate and shake the tube
vigorously. Notice that the precipitate is dissolved in the
ammonium chloride solution and a clear green liquid results.
Nickel phosphate is also soluble in acids. Prepare a solution of 3
measures of sodium bisulphate in a test tube 1/4 full of water and
add to this solution 1/4 of a test tube of the nickel phosphate
precipitate. Notice that the nickel phosphate is again dissolved in
the solution, leaving a clear green liquid.
EXPERIMENT 193 - Copper phosphate
Prepare a solution of sodium phosphate by adding 1/2 measure of
sodium carbonate and 1/2 measure of calcium monophosphate to a test
tube half full of water.
Now prepare a solution of copper sulphate. Add to this copper
sulphate solution a few drops of the sodium phosphate solution and a
very pretty blue precipitate of copper sulphate will be formed.
EXPERIMENT 194-Strontium phosphate
Make a solution of sodium phosphate by dissolving sodium carbonate
and calcium monophosphate in water.
Prepare a solution of 2 measures of strontium chloride in a test
tube half full of water and add to this a few drops of the clear
sodium phosphate solution. A thick white precipitate of strontium
phosphate will be formed.
EXPERIMENT 195 - Aluminum phosphate
Prepare a solution of sodium phosphate as before and add a few drops
of this to a solution of 2 measures of aluminum sulphate in 1/4 test
tube of water.
EXPERIMENT 196-Ferric phosphate
Place 2 measures of ferric ammonium sulphate in a test tube half
full of water and shake to dissolve the solid. Now add to this
solution a few drops of sodium phosphate solution and examine the
brownish white precipitate of ferric phosphate.
96
GILBERT CHEMISTRY
EXPERIMENT I97 - Manganese
phosphate
Dissolve 2 measures of manganese sulphate in a test tube half full
of water by heating. Cool the solution and add a few drops of
sodium phosphate as prepared before. The white precipitate
tinged with pink is manganese phosphate.
EXPERIMENT 198 - Calcium phosphate
Place 2 measures of calcium chloride in a test tube half full of
water and shake vigorously for a minute or two. Now add to
this solution a few drops of sodium phosphate made as before. This
white precipitate formed is calcium phosphate; the same substance
chemically as phosphate rock.
EXPERIMENT 199-Cobalt phosphate
Dissolve 1 measure of cobalt chloride in a test tube half full of
water and add a few drops of sodium phosphate solution. A
beautiful light blue precipitate of cobalt phosphate will appear in
the test tube.
EXPERIMENT 200 - Magnesium
phosphate
Dissolve 2 measures of manganese sulphate [NOTE: This should probably be magnesium sulphate]
in a test tube half full of water. To this solution add a few
drops of sodium phosphate solution prepared as before.
EXPERIMENT 201 - Chromium phosphate
Dissolve 2 measures of chrome alum in a test tube 1/4 full of water.
Fill a second clean test tube 1/4 full of sodium phosphate solution
prepared as before. Now add a few drops of chrome alum
solution to the sodium phosphate solution and a light blue
precipitate of chromium phosphate will form.
THE
ALKALI METALS
The alkali metals are lithium, sodium, potassium and rubidium and
they are all very similar in their chemical behavior. The two most
common are sodium and potassium. These metals are called alkali
metals because they are a part of the compounds which are known as
alkalies or bases, such as caustic soda (sodium hydroxide) and
caustic potash (potassium hydroxide).
Because of their extreme reactivity none of these metals occur free
in nature. They are found as compounds distributed in sea and
mineral water, salt beds and rocks. The free metals are soft,
with a silvery luster, but tarnish quickly in air because of
the reaction with oxygen. They are kept in kerosene out of contact
with the air. They react vigorously with water, setting free
hydrogen from the water.
Some of the more important compounds are sodium hydroxide, the chief
constituent of ordinary lye; sodium chloride, or common salt; sodium
carbonate, or washing soda; sodium bicarbonate, or baking soda.
EXPERIMENT 202 - How to make sodium
hydroxide
Dissolve 2 measures of sodium carbonate in a test tube 1/2 full of
water. To this solution add 2 measures of calcium oxide and
shake the tube. Heat cautiously over the flame for a few minutes to
complete the reaction and filter the solution into another test
tube. The calcium oxide reacted with the sodium carbonate to form
sodium hydroxide and a white precipitate of calcium carbonate. The
clear filtered solution contains the sodium hydroxide.
To a test tube full of water add 3 drops of phenolphthalein solution
and and 2 drops of the sodium hydroxide solution prepared above. The
red color proves that sodium hydroxide is a base. Save the sodium
hydroxide for the following experiments.
GILBERT
CHEMISTRY 97
EXPERIMENT 203 - Manufacture of
sodium bicarbonate or baking soda
Sodium bicarbonate is manufactured on a commercial scale by what is
known as the Solvay process. This consists essentially of
treating a saturated solution of common salt in ammonium hydroxide
with carbon dioxide gas. The carbon dioxide gas reacts with the
ammonium hydroxide to form ammonium bicarbonate which in turn reacts
with the sodium chloride to form sodium bicarbonate. This is
insoluble in the ammonium chloride formed in the reaction and
precipitates out.
Make a saturated solution of common salt by shaking a teaspoonful of
common salt in a flask or bottle with about 3 spoonfuls of common
household ammonia. Filter about a half inch of this strong solution
into a test tube.
Now pass carbon dioxide into this strong solution for half an hour.
Notice the precipitate which forms. This is sodium bicarbonate. The
carbon dioxide is prepared by putting 3 spoonfuls of washing soda or
baking soda in the generator bottle and adding enough water so
that the end of the funnel comes just below the surface of the water
in the bottle. Now add small amounts, 2 or 3 drops at a time, of
vinegar or a solution of tartaric acid to keep up the flow of gas.
EXPERIMENT 204 - To convert sodium
bicarbonate into sodium carbonate
Put 1 measure of sodium bicarbonate into a test tube half full of
water and add 2 drops, no more, of phenolphthalein solution. Notice
the pink color which is produced.
Now heat the solution for several moments and notice that the pink
color changes to red. This is because sodium carbonate which
is formed when sodium bicarbonate is heated has stronger basic
properties than sodium bicarbonate. The gas liberated in the
reaction is carbon dioxide gas.
The hydroxides of many metals are insoluble and are precipitated by
addition of sodium hydroxide to a water solution of salt of these
metals.
HYDROXIDES
EXPERIMENT 205 - Aluminum hydroxide
Dissolve 1 measure of aluminum sulphate in a test tube half full of
water and add a few drops of the sodium hydroxide solution just
prepared. A thick white precipitate of aluminum hydroxide is formed.
EXPERIMENT 206 - Ferric hydroxide
Dissolve 1 measure of ferric ammonium sulphate in a test tube
half full of water and again add a few drops of the sodium hydroxide
solution. A very pretty red-brown precipitate immediately forms
which is iron hydroxide. This material is similar to iron rust
and is used in paints.
EXPERIMENT 207 - Nickel hydroxide
Dissolve 1 measure of nickel ammonium sulphate in a test tube half
full of water, heating the solution a little if necessary, to make
the solid all dissolve. Add a few drops of sodium hydroxide and a
blue-green precipitate of nickel hydroxide is formed.
EXPERIMENT 208 - Manganese
hydroxide
Place 1 measure of manganese sulphate in a test tube half full of
water and shake well to dissolve the solid. Now add a few drops of
sodium hydroxide solution and you will obtain a thick white
precipitate of manganese hydroxide.
98
GILBERT CHEMISTRY
EXPERIMENT 209 - Zinc hydroxide
Place a small piece of zinc and 1 measure of sodium bisulphate in a
test tube half full of water. Warm this solution until the solids
are all dissolved, and after it has cooled a little add a few drops
of sodium hydroxide solution.
EXPERIMENT 210 - Cobalt hydroxide
Dissolve 1 measure of cobalt chloride in a test tube half full of
water and add a few drops of sodium hydroxide solution. A very
pretty blue precipitate of cobalt hydroxide is formed which very
soon changes to red.
EXPERIMENT 211 - Magnesium
hydroxide
Dissolve 2 measures of magnesium sulphate in a test tube half full
of water. Add a few drops of sodium hydroxide solution. A
heavy white precipitate of magnesium hydroxide is formed.
EXPERIMENT 212 - Calcium hydroxide
Dissolve 2 measures of calcium chloride in a test tube half full of
water. Now add a few drops of sodium hydroxide solution and a white
precipitate of calcium hydroxide will form. This calcium
hydroxide is the same substance that is obtained when lime is
slaked.
EXPERIMENT 213 - Chromium hydroxide
Dissolve 2 measures of chrome alum in a test tube 1/4 full of water.
To this solution add a few drops of sodium hydroxide solution. A
beautiful green precipitate of chromium hydroxide is formed.
Potash or potassium carbonate is an important compound and is
essential to plant growth. It is absorbed by plants from the soluble
compounds in the earth or in fertilizers.
EXPERIMENT 214 - Obtaining potash
from wood ashes
Potash was formerly obtained by leeching out the soluble material in
wood ashes with water.
Put 4 spoonfuls of wood ashes in a glass half full of water and stir
well for several minutes. Now allow this mixture to stand for a few
minutes and then filter off a little of the liquid into a test tube.
Test this liquid by adding 2 or 3 drops of phenolphthalein
solution. Notice that the solution turns red. Potassium
carbonate is found in wood ashes and is removed in this way. By
evaporating the liquid down to dryness we could obtain the solid
compound.
ALKALINE
EARTH METALS, CALCIUM, STRONTIUM, BARIUM
The term "alkaline earth" was originally applied to the oxides of
these metals because they resemble both the alkalis and the earths,
the latter term being applied to oxides of aluminum and iron.
These metals do not occur free in nature, but largely as carbonates
and sulphates. They are light and active, resembling each
other closely in physical and chemical properties. They react with
the oxygen in the air and decompose water, liberating hydrogen,
similar to the alkali metals.
Calcium carbonate is found in nature in large quantities as
limestone or marble. Also gypsum (calcium sulphate) and
phosphate rock (calcium phosphate) occur extensively.
GILBERT
CHEMISTRY 99
Lime or calcium oxide is prepared from limestone by heating in large
furnaces or kilns. It is used in making calcium hydroxide and
slaked lime.
EXPERIMENT 215 - Burning limestone
to quick lime
Obtain a few pieces of limestone, marble or oyster shells and break
them up into a powder.
Place some of this powder - 2 teaspoonfuls - on a small tin can lid
and heat over a hot gas flame. Heat so that the limestone
becomes white hot and after twenty minutes' heating allow to cool.
The compound remaining is quick lime or calcium oxide.
Limestone or calcium carbonate, upon heating, decomposes into
calcium oxide and carbon dioxide gas.
EXPERIMENT 216 - Slaked lime or
calcium hydroxide
To a test tube add 1/2 inch of powdered lime and 5 or 6 drops of
water. Notice that the lime puffs up after a few minutes and appears
to be perfectly dry. This forms what is known as slaked lime and is
really calcium hydroxide.
Now fill the test tube 1/3 full of water and shake the contents of
the tube. Filter off the liquid and test it with red litmus paper.
This liquid is a solution of calcium hydroxide. It is a weak
base and is used in medicine as lime water.
Mortar, which is used in stone foundations for buildings, is made by
mixing together water, sand and slaked lime. On exposure to the air
mortar sets or becomes hard. This is because carbon dioxide in
the air reacts with the slaked lime to form an insoluble calcium
carbonate or limestone.
EXPERIMENT 217 - Making mortar
Take three measures of calcium oxide and mix this together
thoroughly with three measures of sand. Then add a few drops of
water to make a paste.
Spread this paste on a board and allow it to stand for several days.
Notice that it soon becomes hard and sets.
Plaster of Paris is made from gypsum or calcium sulphate by heating
until all the water is driven off.
EXPERIMENT 218 - To make Plaster of
Paris
Take a spoonful of calcium sulphate and heat over the alcohol or gas
flame for ten minutes to a high temperature. Allow to cool and then
empty the contents of the spoon on a sheet of paper. This is plaster
of Paris.
EXPERIMENT 219 - Making a cast with
plaster of Paris
Break up the plaster of Paris which you made in experiment 218 into
a fine powder and mix a little of this with water to the consistency
of a paste. Place a coin slightly greased with oil on a paper.
Spread some of this paste on the coin. Press the paste out on the
coin to displace any air bubbles and leave the plaster undisturbed
until hard. Then remove it and you will notice a perfect
imprint of the face of the coin on the plaster.
It is important to call attention to the application of magnesium
chloride and magnesium sulphate in the production of plaster cement
used for stucco construction work. When magnesium oxide is
mixed with strong aqueous solutions of these salts, interreactions
occur with the formation of oxychlorides and oxysulphates,
respectively that have the property of setting like cement. These
cements form the basis of stuccos, flooring, synthetic wood, and
sound absorbing materials. This material has found a very extensive
use for the construction of office buildings and public buildings.
100 GILBERT CHEMISTRY
Calcium sulphide when it contains traces of sulphides of some of the
other metals has the property of absorbing light from a luminous
body and then giving up light in the dark. It is prepared by heating
calcium sulphate with carbon.
EXPERIMENT 220 - Calcium sulphide
light
Expose a piece of calcium sulphide paper to a gas or electric light
for several minutes. Now take the paper into a dark room and
notice that it is luminous and gives off light.
Certain metals or their compounds impart a characteristic color to a
flame. While the alkaline earths resemble each other in chemical
properties, they differ in the colors they give to a flame. Calcium
compounds give a red flame, strontium gives carmine and barium gives
a green flame.
The alkali metals and their compounds also give characteristic
colors in a flame. Sodium gives a yellow flame and potassium a
purple flame. Copper compounds give a green flame.
EXPERIMENT 221 - Flame test for
metals
Clean the steel wire and make a loop in the cleaned wire. Heat the
loop in a flame until there is no color given to the flame by the
wire.
Dip the clean wire into some powdered calcium chloride and then heat
in the tip of the flame. Notice the red flame of calcium.
EXPERIMENT 222 - Red fire
Mix together thoroughly in a pan one measure of strontium nitrate,
two measures of potassium nitrate, one measure of sulphur and two
measures of powdered charcoal. Make into a small pile and keeping
the face at a safe distance light the pile with a match. Notice that
the mass takes fire readily and burns with a red light, due to the
strontium. The sulphur and charcoal act as combustible materials,
while the potassium nitrate furnishes oxygen for the reaction.
EXPERIMENT 223 - How to make green
fire
Zinc, when in the form of a powder, burns with a green flame.
Repeat experiment 222, using two measures of powdered zinc, two
measures of potassium nitrate, two measures of powdered charcoal and
one measure of sulphur. Notice that this mixture when ignited
will burn with a green flame. These mixtures can be ignited to good
advantage by the use of a fuse. The fuse is prepared by soaking a
piece of ordinary string in a strong solution of potassium nitrate
and allowing the string to dry.
EXPERIMENT 224 - How to make yellow
fire
Sodium when burned produces a yellow flame.
Repeat experiment 223, using one measure of dry sodium chloride, two
measures of potassium nitrate, one measure of sulphur and two
measures of powdered charcoal. Ignite the mixture and notice
that it burns with a yellow flame.
EXPERIMENT 225 - Preparing a safe
explosive
Chemicals needed: iodine crystals and ammonia. Apparatus you will
need: test tube and filter paper.
Crush several of the iodine crystals and place them in a clean test
tube. Pour in about one teaspoonful of ammonia and let this
stand for half an hour. Then filter it. Do not let
filtrate spill on the table. Dry the filter paper in dry open
air. As soon as it is dry just a scratch on the filter paper will
explode the crystals.
"The Science Notebook"
Copyright 2008-2018 - Norman Young
 The
Science Notebook
The
Science Notebook The
Science Notebook
The
Science Notebook