 The
Science Notebook
The
Science NotebookGilbert Mineralogy - Part I
Home Terms of Use Safety Contact Us
Experiment
Pages Downloads
Supplies
Useful
Links!

 The
Science Notebook
The
Science Notebook Home Terms of Use Safety Contact Us
Experiment
Pages Downloads
Supplies
Useful
Links!


As you may know, naturally occurring materials are commonly divided into three kingdoms; that is, animal, vegetable, and mineral. Minerals are those materials that make up the bulk of the earth's crust and are called inorganic materials because they have not been made by any of the processes of life. A mineral may be described as a naturally occurring substance of, definite and uniform chemical composition and of characteristic physical properties. Mineralogy is that branch of science which has for its object the accurate investigation of these naturally occurring products as regards their physical and chemical properties, their economic importance and their uses in the arts.
Of the naturally occurring inorganic products two classes are recognized, and. these are known as minerals and rocks. Minerals, as we have already stated, are definite chemical compounds. A few of the following minerals with their chemical name and formula may serve as examples:
There are today between eight and nine hundred minerals known, although only a few are common minerals and important as rock making minerals, as ores of the useful metals or otherwise important in the industries.
Rocks are often used in a general way for designating any portion of the earth's crust. Rocks, however, contain certain minerals. For example, the rock known as granite contains the minerals, orthoclase KAlSi3O8, albite NaAlSi3O8, quartz SiO2 and quite often several others in small amounts. Sometimes rocks consist of only one mineral, for example, quartzite. This is a rock containing only one mineral, namely, quartz SiO2.
Since minerals have a definite chemical composition which is known to be true by chemical analysis, it is often easy to distinguish between the different species of minerals. Many minerals differ from each other in their composition, and consequently minerals behave quite differently when treated with the various chemical reagents. These differences in behavior when minerals are treated with chemical reagents furnish one important means of identifying minerals and determining the percentages or the amounts of the different constituents which go to make up the mineral.
The other important means of identifying minerals has to do mainly with the physical properties of minerals. Minerals differ widely in their physical properties, such as crystallization, luster, color, hardness, fusibility and specific gravity, and
consequently it is quite often possible to identify a mineral by applying some of the common physical tests. The method of identifying minerals by means of chemical reagents is known as chemical mineralogy, while that of making use of the physical properties of minerals is called physical mineralogy.
There is a third means by which minerals may often be identified, and this branch of mineralogy is known as Crystallography or Crystallographic mineralogy. Many minerals have their own definite crystal forms by which they may very readily be recognized. These forms are known as cubes, prisms, hexagons, pyramids, etc. It is only when minerals are found occurring in crystalline form that this method of identification can be used. Since many minerals are not found in the crystalline form, it is necessary in these cases to identify the mineral by other physical or chemical means.
The question which naturally comes up in every boy's mind is, “What is the value of the science of mineralogy; how can it be shown to be important not only as a branch of science but important economically or from a business standpoint?” The answer to this question is very easily understood when‘ you realize that today the prospector and mining man depend upon the economic mineralogist in searching for mines or handling ores and minerals. Sometimes valuable mines or worthless ones are reported on unfavorably by men not familiar with the principles and details of mineralogy. Again large sums of money may be lost by wrongly carrying out certain operations such as ore-dressing. It can be seen, therefore, that a knowledge of mineralogy - especially economic mineralogy - is a very important asset to the miner, prospector and metallurgist. This is the bigger aspect of the importance of mineralogy as a science. The other aspect, and one which ought to appeal very strongly to boys, is the fun derived from knowing how to identify or spot on sight different minerals as you find them, either alone or in the rocks in nature. As you become acquainted with the habits, forms, properties and peculiarities of the different minerals you will be able to picture all of these things in your mind on finding some of the minerals in nature. You will become much more interested in nature and will really see more things which before meant practically nothing but which are now of fascinating interest to you. Then again you may come across a mineral which you are not able to identify by sight. You cannot appreciate the fun there is in applying chemical and physical tests to these minerals and establishing for yourself the name of the unknown mineral.
It is suggested that you read over carefully Part I and Part II
and become acquainted with the principles and terms of mineralogy
before you attempt to examine the minerals described in Part III.
By doing this you will understand more clearly the meaning of the
different terms as used in the description of minerals and will be
better able to obtain accurate results in the identification of
the minerals.
Elements are substances which up to the present time have defied all attempts of the chemists to decompose them. More than eighty elements are now known, but many of these are rare and only of scientific interest. It has been calculated that 99 per cent of the crust of the earth, for a depth of ten miles, is composed of the following eight elements:
| Oxygen Silicon Aluminum Iron Calcium Magnesium Magnesium Sodium Potassium |
47.3 per
cent 27.2 " " 7.8 " " 5.4 " " 3.8 " " 2.7 " " 2.7 " " 2.4 " " 2.4 " " |
The elements like gold, lead, platinum, copper, silver, etc., which are not included in this list but which are of economic importance only make up about one per cent of the earth's crust.
By Chemical Affinity is meant the power or tendency that elements have to unite with one another. This tendency of uniting is strongest between metallic and non-metallic elements, such as sodium and chlorine in sodium chloride (common table salt).
An Atom is the smallest particle of an element that enters into chemical combination. An atom is so small that you cannot see it under the most powerful microscope.
A Molecule is the smallest particle of a chemical compound that is capable of existing. Molecules may consist of atoms of one kind; for example, a molecule of oxygen consists of two atoms of oxygen. Or Molecules may consist of atoms of different kinds; for example, a molecule of the mineral galena consists of one atom of lead and one atom of sulphur.
The Law of Definite Proportion means that atoms unite with each other in definite proportions,although the one atom may unite with another atom in two or more different proportions. For example, carbon forms two distinct oxides with oxygen, one called carbon monoxide (CO), in which one atom of carbon unites with one atom of oxygen, and the other called carbon dioxide (CO2), in which one atom of carbon unites with two atoms of oxygen.
Symbols are used for sake of convenience for designating an atom of an element. They are usually the initial letter of the English or Latin name of the element. For example, O is the symbol for an atom of oxygen and Pb is the symbol for one atom of lead (Latin plumbum).
Formulae are used to designate a molecule of a substance. For example, O2 is the formula of and represents one molecule of oxygen, or PbS the formula of and represents one molecule of galena or lead sulphide.
The Atomic Weight of an element is the weight of an atom of the element compared with the weight of an atom of hydrogen, which is unity and the lightest element known.
The Molecular Weight of a substance is the sum of the atomic weights taken as many times as they occur in,the molecule. For example, the atomic weight of lead is 207, of sulphur 32; therefore the molecular. weight of lead sulphide is (207+32) = 239.
By Valency is meant the combining power of an atom of an element, or in other words the number of atoms of an element which will unite with or replace one atom of hydrogen. For example, chlorine is monovalent, that is, it combines with one atom of hydrogen to form the molecule HCl, hydrogen chloride. Oxygen is bivalent, that is, it combines with two atoms of hydrogen to form a molecule of H2O (water).
Acids are compounds which are formed by the union of hydrogen with a non-metal or group of non-metallic elements in which the hydrogen atoms may be replaced by metals. Acids have a sour taste and turn blue litmus red. These properties are due to the replaceable hydrogen (H) which they contain. HCl hydrochloric acid, H2SO4 sulphuric acid and HNO3 nitric acid are a few examples of some of the common acids.
Bases are compounds formed by the union of metals with hydrogen and oxygen. They are also called alkalies or hydroxides. They have a soapy taste and turn red litmus blue. These properties are due to the (OH) hydroxyl group which they contain. NaOH sodium hydroxide, KOH potassium hydroxide and Ca(OH2) calcium hydroxide are a few examples of some of the common bases.
Salts are compounds formed by the action of an acid on a base in which part or all of the hydrogen of the acid is replaced by the metal of the base. For example, the action of sodium hydroxide (NaOH) on hydrochloric acid (HCl) produces the salt sodium chloride NaC1 and water H2O. Similarly, the salt sodium sulphate would be formed by the action of sulphuric acid on sodium hydroxide. Most of the minerals are salts and are therefore classified in groups according to the acid radical which they contain; for example, the silicates, salts of silicic acid in one group, the sulphates, salts of sulphuric acid in another group, and so on.
Oxides are compounds formed by the union of oxygen with metals or non-metals. For example, the union of the metal aluminum with oxygen gives a metallic oxide called aluminum oxide and is found in nature as the mineral corundum (Al2O3). Other examples are tin oxide known as tinstone (SnO2) and silicon dioxide, known as quartz (SiO2). On the other hand, non-metallic oxides are formed by the union of non-metals like sulphur, phosphorous, carbon, etc., with oxygen. Sulphur dioxide (SO2) and carbon dioxide (CO2) are some examples of non-metallic oxides.
By Oxidation is meant a chemical change in which oxygen is added to an element or compound. For example, when the metal zinc is heated in the air it takes up oxygen from the air to form a white compound, zinc oxide. This may be expressed in the form of an equation as follows:
Reduction on the other hand is applied to a chemical reaction in which oxygen is removed from a compound. For example, if hydrogen, is passed over hot zinc oxide the oxygen is removed by the hydrogen to form water, the other product being metallic zinc. This reaction may be expressed as follows :
ZnO + H2 = H2O + ZnIt can be seen from the above that oxidation is the opposite of reduction. It is important to get these two chemical changes clearly in mind, as they are of very great importance in the blowpipe analysis of minerals.
A Chemical Equation is a way of expressing in an abbreviated form what takes place in a chemical reaction when substances react with or unite with one another. For example, when the mineral calcite, calcium carbonate (CaCO2)3, is dissolved in hydrochloric acid (HCl) the reaction may be expressed very clearly in the form of an equation as follows:
CaCO3 + HCl = CaCl2 + H2O + CO2Water-of-crystallization is the water combined with a compound when it crystallizes out in nature. This water can usually be driven off from the compound by heating it. For example, the mineral gypsum as it is found in nature contains two molecules of water-of-crystallization. Similarly the mineral borax contains ten molecules of water.
In determining what elements are contained in an unknown mineral and in what amounts, the mineralogist employs what is known as a qualitative and quantitative analysis. He first runs a qualitative analysis on the mineral and in this way finds what the elements are which go to make up the mineral. He then runs a quantitative analysis, which gives him the necessary data for figuring out the percentages of the different elements in the mineral. From the percentages of the different constituents he can readily calculate by means of the atomic weights of the elements the ratio of the constituents, and in this way establish the formula for the unknown mineral. This is the manner in which the formulae for all the known minerals have been worked out.
The blowpipe is a very useful and convenient instrument for
making several tests on minerals. It consists essentially of a
tube with a very small opening at one end through which air can be
forced from the lungs in a small stream under pressure. By means
of the blowpipe it is possible to convert a small luminous flame
into a very hot flame, and in this way many important tests can be
made.
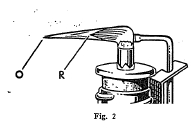
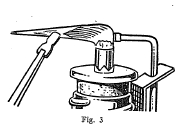
Some minerals when heated alone on charcoal by means of the oxidizing flame of the blowpipe give characteristic oxide coatings. Other minerals when mixed with powdered charcoal and sodium carbonate and heated in the reducing flame on charcoal are reduced with the formation of globules of the metal present in the mineral.
The following table gives a list of the elements which give characteristic oxide coatings when their minerals are heated alone in the flame on charcoal:
| ELEMENT | COLOR AND CHARACTER OF COATING |
| Lead | Yellow near mineral and white farther away. Coating is due to formation of lead sulphite and sometimes lead sulphate |
| Zinc | Yellow when hot and white when cold. Coating is very close
to the mineral and is non-volatile in the oxidizing
flame. Coating turns green when moistened with cobalt
nitrate and heated intensely. |
| Antimony | White. Coating is volatile and is deposited close to the mineral. |
| Arsenic | White. Coating is volatile and is deposited away from the mineral. Usually accompanied by garlic odor. |
| Bismuth | Yellow near mineral and white farther away. Distinguished
from lead-oxide coating by fusing on charcoal with potassium
iodide and sulphur. Coating is yellow near mineral and
brilliant red farther away. Under similar conditions lead
would give a solid yellow coating. |
| Molybdenum | Light yellow when hot and white when cold. Coating is
non-volatile in the oxidizing flame. Coating becomes dark
blue when heated for a second in the reducing flame. |
| Sulphur | Odor of burning sulphur or sulphur dioxide. |
The following table given a list of the important elements which give metallic globules when their minerals are mixed with powdered charcoal and sodium carbonate and heated in the reducing flame on charcoal:
| Silver | Silver-white globule - malleable. |
| Lead | Soft metallic globule - malleable - easily fused and marks paper. |
| Bismuth | Silver-white globule - brittle. |
| Tin | Tin-white globule - soft and malleable - does not mark paper. |
| Gold | Yellow globule - soft and malleable. |
| Copper | Red spongy mass. |
| Nickel | Residue - slightly magnetic. |
| Cobalt | Residue - slightly magnetic. |
| Iron | Residue - slightly magnetic. |
Nickel-steel wire, which is an alloy of the metals nickel and
steel, may be used in place of platinum for making certain tests
in mineralogy. (See Fig, 6.)
Some metals when dissolved in certain compounds called fluxes give a characteristic color to the fused mass. Borax and sodium carbonate are two compounds which are used as fluxes. By means of these color reactions it is quite often possible to tell what metal is present in a mineral.
One end of the wire is bent into a loop, which should he about 1/4 of an inch long and 1/8 of an inch w1de. The loop is first heated in the blowpipe flame, dipped into the flux which is then fused into a head on the loop by heating again in the flame. A small amount of the mineral about the size of a pin head is now placed on the bead and the head heated in the flame until the mass is well fused. On cooling, the characteristic color of the metal present in the mineral will be imparted to the bead. The color of the bead will depend on whether it was heated in the oxidizing or reducing flame. The following table given a list of the important bead tests of several of the metals:
| ELEMENT | OXIDIZING FLAME | REDUCING FLAME |
| Copper | Blue | Opaque red |
| Iron | Yellow | Bottle-green |
| Manganese | Reddish-violet | Colorless |
| Cobalt | Deep blue | Deep blue |
| Chromium | Green | Green |
| Nickel | Reddish-brown | Opaque gray |
| Uranium | Yellow | Pale green |
Certain elements give characteristic flame color tests when
heated on nickel-steel wire in the blowpipe flame. One end of the
wire is first cleaned by scouring it with sea sand. It is then
heated in the blowpipe flame until the flame from the wire becomes
nearly colorless. The wire is then touched to some of the finely
pulverized mineral, and then introduced into the blowpipe flame.
The following table gives a list of the important elements with
their flame colors:
| ELEMENT | COLOR OF FLAME |
| Copper | Emerald green from oxide of copper. |
| Copper | Azure blue from chloride of copper |
| Sodium | Yellow |
| Strontium | Crimson |
| Lithium | Crimson |
| Calcium | Orange |
| Barium | Yellow-green |
| Boron | Yellow-green |
| Zinc | Bluish-green |
| Molybdenum | Pale azure-blue |
| Molybdenum | Yellow-green |
Where two or more elements give the same color flames other tests have to be applied in order to satisfactorily distinguish between these elements.
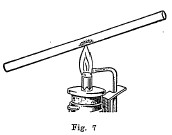
Open and closed tubes are often used for testing materials. The open tube (Fig. 7) should be from 6 to 8 inches in length and have an inside diameter of about 1/4 of an inch. This tube is used ordinarily for making oxidation tests. A small amount of the powdered mineral is placed in the tube about one-third of the way by using a narrow strip of paper folded together to serve as a boat for introducing the mineral. The tube is then inclined slightly and heated gently at first at a point just above the mineral, and finally directly under the mineral. By heating in this manner, the mineral is oxidized by a current of air which passes up through the tube. In most cases a deposit called a sublimate will be formed on the upper, cooler portion of the tube. It is often possible to tell by the nature and color of this sublimate what metal is present in the mineral. Again a gas may be formed by oxidation, the odor of which makes it possible to tell one or more of the elements which are present. The following table gives a list of the elements and a description of their behavior when heated in an open tube:
| ELEMENT | BEHAVIOR ON HEATING |
| Sulphur | Characteristic fumes of burning sulphur or sulphur dioxide. A piece of moistened blue litmus paper held over the upper end of the tube turns red due to the acid reaction. |
| Antimony | Volatile white ring or sublimate close to the mineral. |
| Arsenic | Volatile white sublimate far from the mineral. Odor of
garlic |
| Mercury | Forms minute gray globules of metallic mercury. |
| Molybdenum | Pale yellow sublimate close to the mineral. |
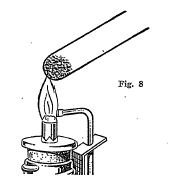
Closed end tubes (Fig. 8) are easily made by heating a piece of soft glass tubing about 8 inches long in the center and pulling it apart when soft. A little of the powdered mineral is then introduced into the tube and the tube heated over a hot flame. Heating a mineral in a closed tube prevents the mineral from reacting with oxygen from the air. In this way the mineral ordinarily breaks down into simpler parts or fuses together. The following table gives.a list and description of some of the important closed tube tests:
| SUBSTANCE | TEST |
| Water | Minerals containing water-of-crystallization will give a
deposit of minute drops of water on the upper portion of the
tube. |
| Sulphur | Minerals containing an excess of sulphur give an orange
colored sublimate when hot and a yellow sublimate when cold.
|
| Arsenic | Arsenic and arsenic containing sulphur gives a reddish-yellow sublimate when cold and deep red when hot. |
| Antimony | Antimony containing sulphur gives a brownish red sublimate
when cold and a black sublimate when hot. |
| Mercury | Mercury containing sulphur gives a black sublimate. When heated with sodium carbonate and charcoal gives globules of metallic mercury. |
In Part I minerals were regarded from the standpoint of their chemical properties. They also possess certain physical properties which may be very useful for their identification and recognition. Some of the most important of these are structure. cohesion. tenacity, hardness, luster, color and specific gravity. It is important to have clearly in mind the meaning of these physical properties before endeavoring to make a study of any of the common minerals.
Granular - When a mineral consists of grains either fine or coarse as marble and some forms of galena.
Massive - A mineral composed of compact material with an irregular form. It does not have any crystal faces but may possess crystalline structure. For example, some varieties of quartz, chalcopyrite, etc.
Compact - Earthy - A mineral consisting of a uniform mass of very minute particles.
Amorphous - Minerals possessing no trace of crystalline structure.
Columnar - Minerals possessing a column like structure usually in parallel groupings of prisms or columns. For example. some varieties of hornblende, beryl and wolastonite.
Fibrous - Minerals consisting of fine thread-like strands which may be separated or pulled apart. (Fig. 12). Illustrated by such minerals as serpentine, amphibole (asbestos) and gypsum (satin-spar).
Foliated - A mineral which may easily be separated into plates; for example, some varieties of serpentine and brucite.
Micaceous - Similar to foliated minerals but in which the mineral can be easily split into very thin sheets like muscovite (common mica).
Reniform and Mammillary -
Kidney shaped minerals, such as some varieties of hematite and
malachite. (Fig. 14)
Stalactitic - Minerals having icicle like forms an usually found in the roof of some cavity and formed from dripping water. For example, some varieties of limonite and calcite. (Fig. 16).
Cleavage - A mineral us said to possess cleavage when it shows definite plane surfaces resembling crystal faces when the mineral is broken. (Fig. 17).
The directions of cleavage are always parallel to some crystal surface. Only a few minerals possess perfect cleavage, whale many do not possess any cleavage and in others the cleavage is poor. The cleavage of a mineral is described according to the crystal face to which it is parallel; for example, cubic cleavage (halite and galena), octahedral cleavage (calcite), dodecahedral (sphalerite), prismatic cleavage (amphibole), basal cleavage (topaz) and pinachoidal cleavage (stibnite).
Parting - Some minerals when subjected to a strain break or part along certain plane surfaces. When minerals exhibit this property they are said to have a parting. It resembles cleavage somewhat but differs from it in that cleavage can take place as readily in one part of crystal as in another.
Magnetite sometimes shows a perfect octahedral parting but has
no apparent cleavage. (Fig. 18)
Minerals possess certain properties which depend upon their tenacity. The following terms are used to describe the various kinds of tenacity:
Brittle - When a mineral crumbles or powders easily. Example: Iron pyrites, apatite, fluor spar, etc
Ductile - When a mineral can be drawn out into a wire. Example: native metals.
Flexible - When a mineral bends but does not resume its former shape when the pressure is released. Example: talc, selenite, etc.
Elastic - When a mineral, after being bent, will spring back to its former position. Example: mica.
Malleable - When a mineral can be flattened out into thin sheets under a hammer. Example: native silver, gold, copper, platinum, etc.
Sectile - When a mineral can be cut into thin shavings with a knife. Example: graphite, steatite, etc.
Minerals vary widely in their hardness. By hardness is meant the resistance which a mineral offers to being scratched. A scale of hardness is usually represented by crystallized varieties of the following minerals:
The finger nail is a little over 2 in hardness, since it scratches gypsum but not calcite. A cent is about 3 in hardness, for. it just scratches calcite. An ordinary pocket knife is just over 5, and window glass a little harder, about 5.5.
Sometimes a softer mineral will leave.a mark on a harder mineral and this must not be mistaken for a scratch. A mark made in this manner can be easily rubbed off while a scratch will be permanent. On testing a mineral for hardness always use a fresh surface of the mineral as an old surface may be somewhat changed and softer than a fresh surface.
The term luster as applied to minerals is the appearance of the mineral due to the effect of light upon it. Minerals come under one of three classes: those that have metallic luster, those that have a non-metallic luster and those that have a sub-metallic luster.
By metallic luster is meant having the appearance of a metal. Most minerals that come under this class are opaque and their powders are black or dark colored. Examples: galena and pyrite. The luster of a mineral is usually determined by rubbing the mineral across a piece of unglazed white porcelain. The mineral leaves a streak which gives the color of the finely powdered mineral.
Minerals with a non-metallic luster are transparent to light on their thin edges. These minerals are in general light colored and their streaks are colorless or very light colored. The following terms are used to further describe the appearance of minerals having a non-metallic luster:
Adamantine - Hard, brilliant luster like the diamond.Color is a very important property of minerals and serves as an important means of identification. Since the color on some mineral surfaces changes somewhat on long exposure, it is always essential to examine a fresh surface of the mineral before noting the color. The color of a mineral is a very definite and constant property. For example, the black color of magnetite, the bluish-gray of galena, the brass yellow color of chalcopyrite, and the green of malachite are all characteristic colors of these minerals. Many minerals vary in color, however, in different specimens. This is due sometimes to impurities in the mineral. For example, sphalerite is sometimes brown and black, due to the presence of iron as an impurity. Again quartz which is usually colorless may be colored red, due to the presence of a small amount of hematite. Fluorite is found in many different colors, such as white, pink, yellow, green and blue.
By specific gravity of a mineral is meant the relation between the weight of the mineral and the weight of an equal volume of water. For example, quartz is known to have a specific gravity of 2.65, which means that quartz is 2.65 times as heavy as the weight of an equal volume of water. In obtaining the specific gravity of a mineral it is essential that the mineral be pure and crystalline, otherwise the specific gravity will vary. If the mineral contains cracks or holes, it is necessary to boil the mineral in water for several minutes to displace the air contained in the cracks, as the air tends to make the mineral lighter.
The method usually applied for obtaining the specific gravity of a mineral is to weigh the mineral in air and then in water. The differences in weight is the weight of a quantity of water equal to the volume of the mineral, because when a body is immersed in water it is buoyed up by a weight which is equal to the weight of the water displaced. If (x) is the weight of the mineral in air and (y) is the weight when immersed in water, then the specific gravity may be expressed as follows:
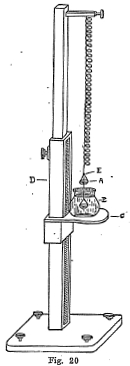
platform C. On the front side of the upright D there is a
mirror and graduated scale. There is a small bead at E which
serves as a mark for noting the position of the spring in regards
to the graduated scale.
Three readings are taken: first, the position of the balance
in regards to the scale with the lower pan in the water; second,
the position of the balance in regards to the scale when the
mineral is placed in the upper pan or when weighed in the air;
third, the position of the balance when the mineral is placed in
the lower pan or when the mineral is immersed in water. The
difference between the first and second reading gives the weight
of the mineral in air and the difference between the first and
third reading gives the weight of the mineral in water. From these
two weights the specific gravity can readily be calculated, using
the formula described on the previous page.
There are a few of the common minerals which are attracted by a magnet. Some minerals containing iron, nickel and cobalt are attracted by a strong electro-magnet, and this test sometimes affords a convenient means of recognizing these minerals. Other minerals are attracted only after strong heating (ignition) in the air. This is also used as a valuable test in recognizing certain minerals.